We offer consulting and solutions to assist with the complicated process of designing medical devices. Use our experience to find solutions to technical and compliance issues that arise during the product development process. We have been involved in the development of dozens of medical devices including software controlled electrosurgical generators, battery-operated disposalbe products and ESU accessories. Let our experience provide solutions to keep projects on schedule and save money by avoiding common mistakes and assumptions. The medical device market is constantly changing, stay ahead of the curve!

We offer the following Design and Development Services:
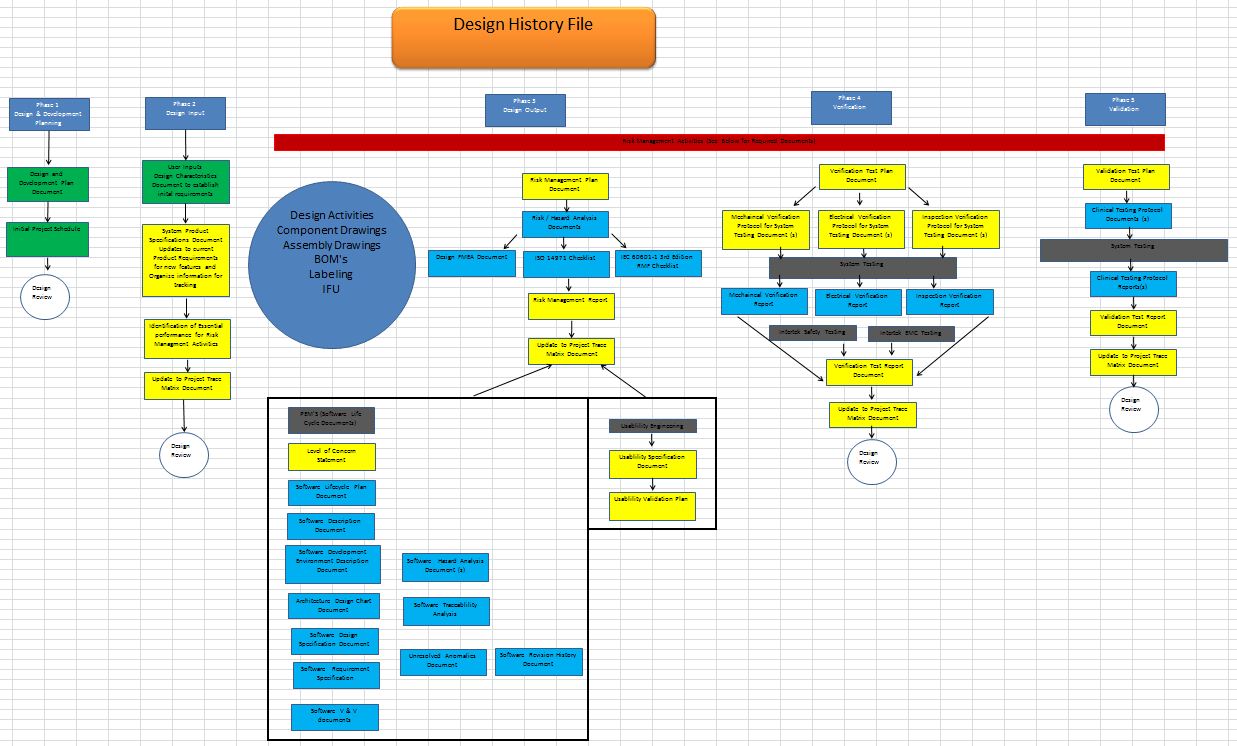
We work with customers to fulfill quality system requirements to ISO13485 including specific customer requirements. Services include:
We provide verification testing to required medical device standards. Product requirements are reviewed and verification testing is developmed to meet device specific requirements. Can work with external testing agencies to communicate customer's need for EMC, Safety, Usability, Software / PEM.
Particularly experienced in the following standards:
ANSI/IEC 60601-1:2005 3rd Edition
ANSI/IEC 60601-2-2:2009